Instrumentation used for photochemical research
PhotoCube photoreactor
 | Conventional, flow and "stop-flow" chemical reactions can be performed in the photoreactor, with excitation light of several wavelengths (365, 395, 457, 500, 523, 595, 623 nm and white light). The LED power can be changed up to 128 W for each color. Several wavelengths can be used simultaneously. The reactor space can be thermostated in the range of 20-80 °C. |
Top of page
Diode-array spectrophotometer as a photoreactor
 | In a diode-array spectrophotometer (like the computer controlled AnalytikJena SPECORD S600 or HP-8543), a high intensity polychromatic light beam passes through the sample with enough light intensity to induce photochemical reactions. The main adventage of the method is that following the reaction by sectrophotometry is obvious.
|
Top of page
Diode-array spectrophotometer with external lamp
In a diode-array spectrophotometer (like the one in our research group, a computer controlled (C) AnalytikJena SPECORD S600 (B)), the induction of photochemical reaction is even more effective when using a high intensity external lamp. Our external lamp is either a Spectroline FC-100/F UV-A lamp emitting at 365 nm (A) or a 500 W NINGBO OALY halogen lamp (D). When used in the arrangement on the photo, they have a photon flux one order of magnitude higher as the photon flux from the internal lamps of the spectrophotometer in the whole (190-1100 nm) wavelength range.
|
Top of page
Photochemical reactor combining a CCD spectrophotometer and a LED
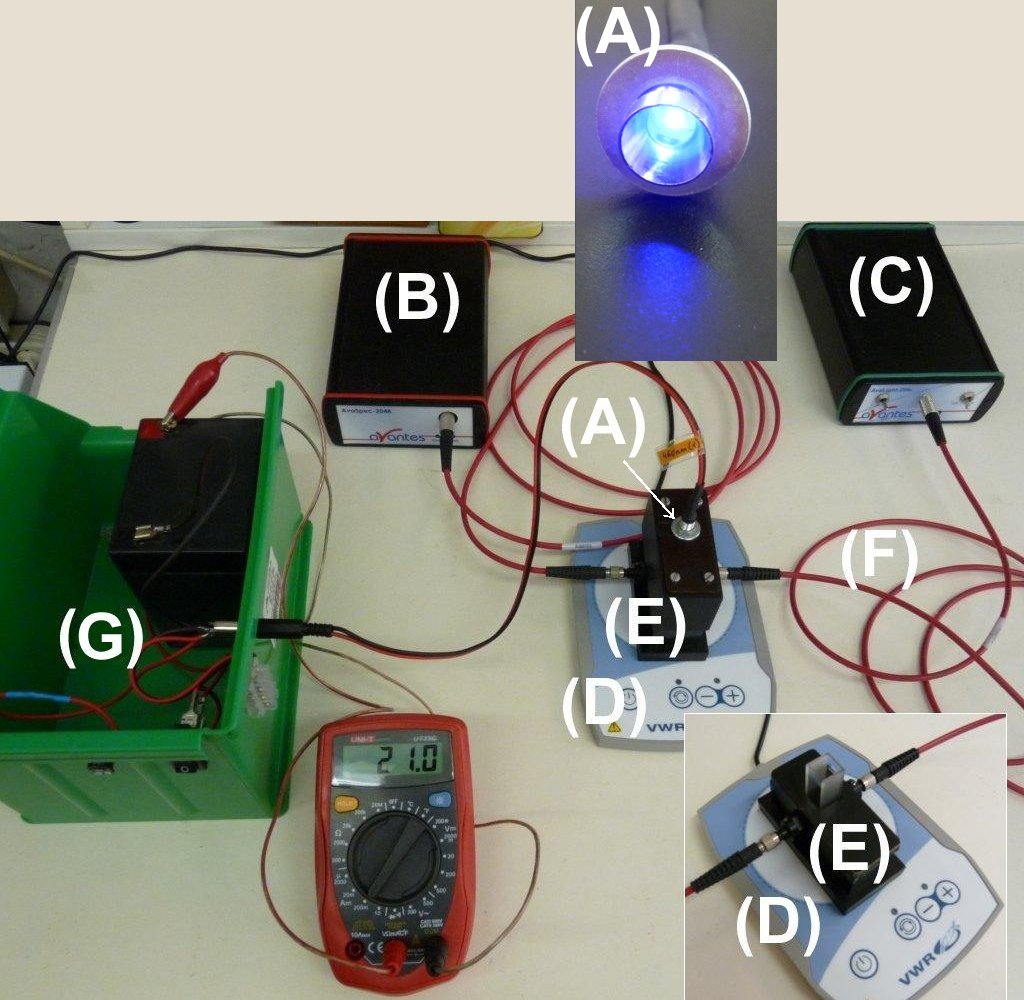
| In the photochemical reactor made of brass (E), the photoreaction is induced from the top by a quasi-monochromatic LED irradiation source (A) and it is followed by an Avantes fibre-optic spectrophotometer (optical fibres: (F); spectrophotometer lamps: (C); CCD detector: (B)). The sample is placed into a normal, 1,000 cm pathlength cuvette (on the small Figure on the right-bottom, the reactor is opened so the cuvette can be seen). Because of the brass material of the reactor, the sample can be stirred with a magnetic stirrer (D).
The power supply (G) of the LED can be controlled by a computer making possible to use different illumination profiles. Also, there is the possibility of illuminating the sample independently of the detecting light beam (Ref. 1).
|
Top of page
Photochemical reactor combining a CCD spectrophotometer and an external lamp
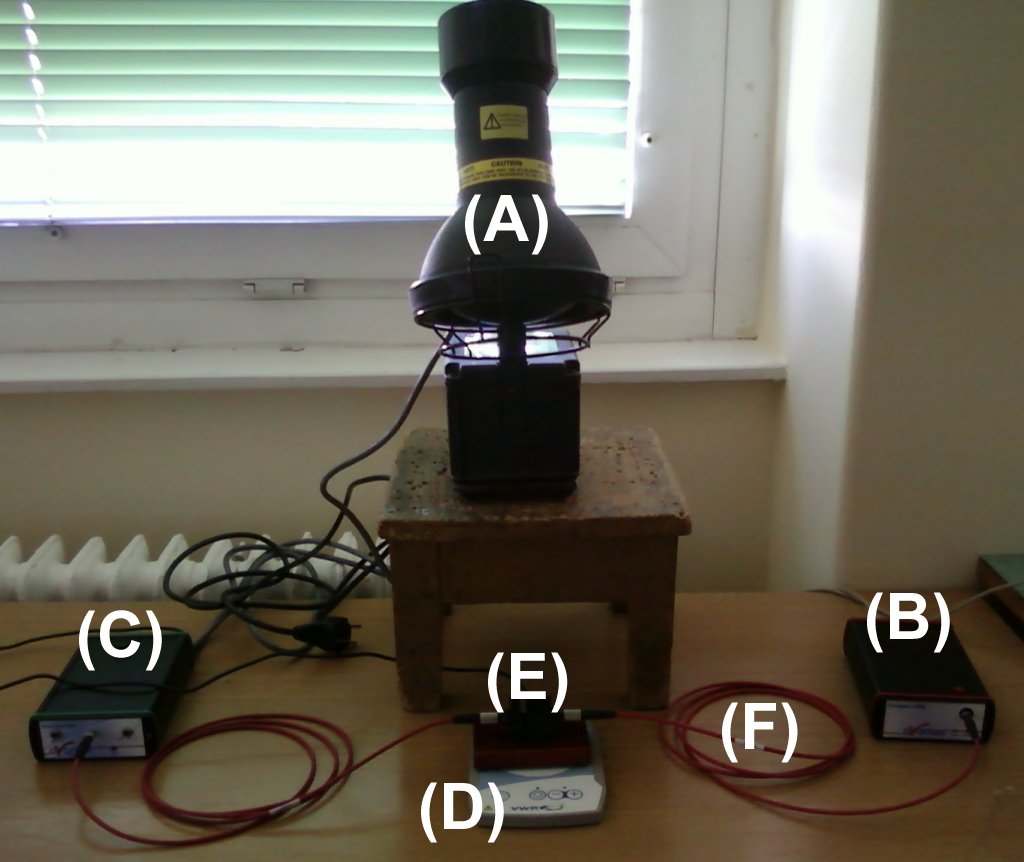
| The photochemical reactor introduced in the previous paragraph can be used not only with LED but also with external Spectroline FC-100/F UV-A lamp radiation source (A) emitting at 365 nm, or a 500 W NINGBO OALY halogen lamp emitting in the visible wavelength range. The reaction is still followed by an Avantes fibre-optic spectrophotometer. Parts of the photoreactor and other complementaries (B-F) are the same as on the previous photo. |
Top of page
Automatic titrator with external lamp
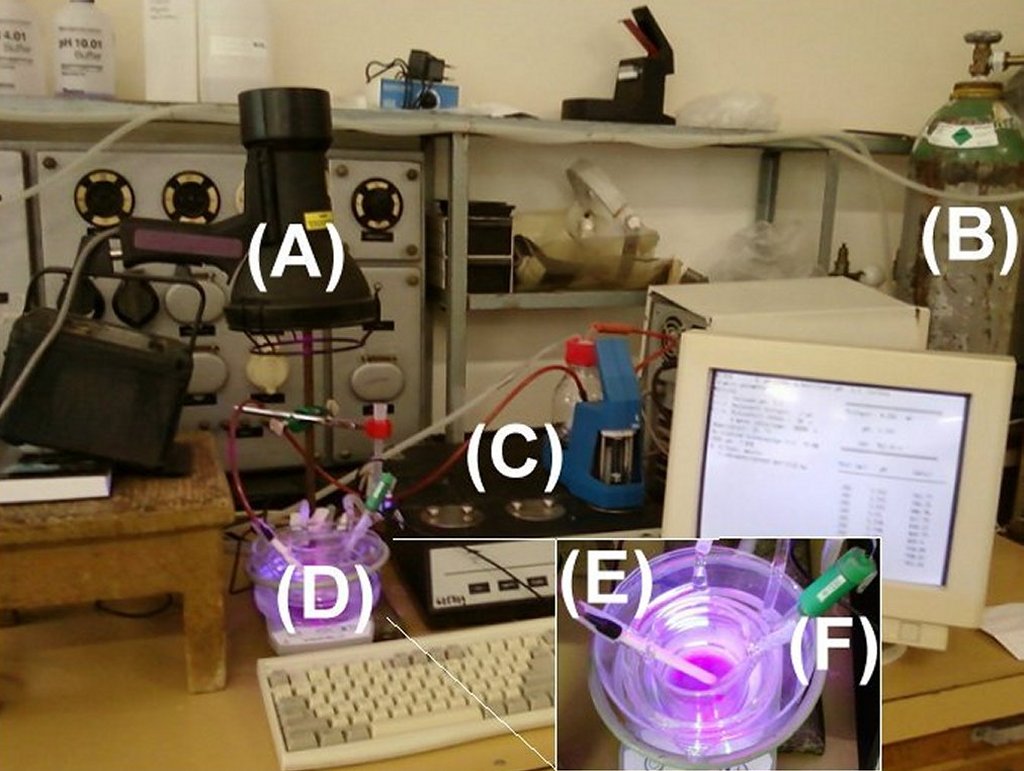
When at least one of the products of a photochemical reaction is acid, then the kinetics of the acid formation can be followed by a continous back-titration of the acid formed in the process (e.g. by a pH-stat measurement, keeping the pH of the solution constant and adding the required amount of base to the solution). (Ref. 1: photochemical formation of hydroxy-quinones and hydroquinones (weak acids) from 1,4-benzoquinones). To follow the kinetics of the acid formation, a computer controlled automatic titrator system (C) is used. The illumination source is the Spectroline FC-100/F UV-A lamp emitting at 365 nm (A), or a 500 W NINGBO OALY halogen lamp emitting in the visible wavelength range. The reaction wessel (D) can be thermostatted. A base tube (E) and a pH-selective glass electrode (F) is immersed into the sample. To avoid carbonation, Ar gas (B) is used. The sample is mixed using a magnetic stirrer.
|
Top of page
Sample holder for measurements with light sensitive electrodes
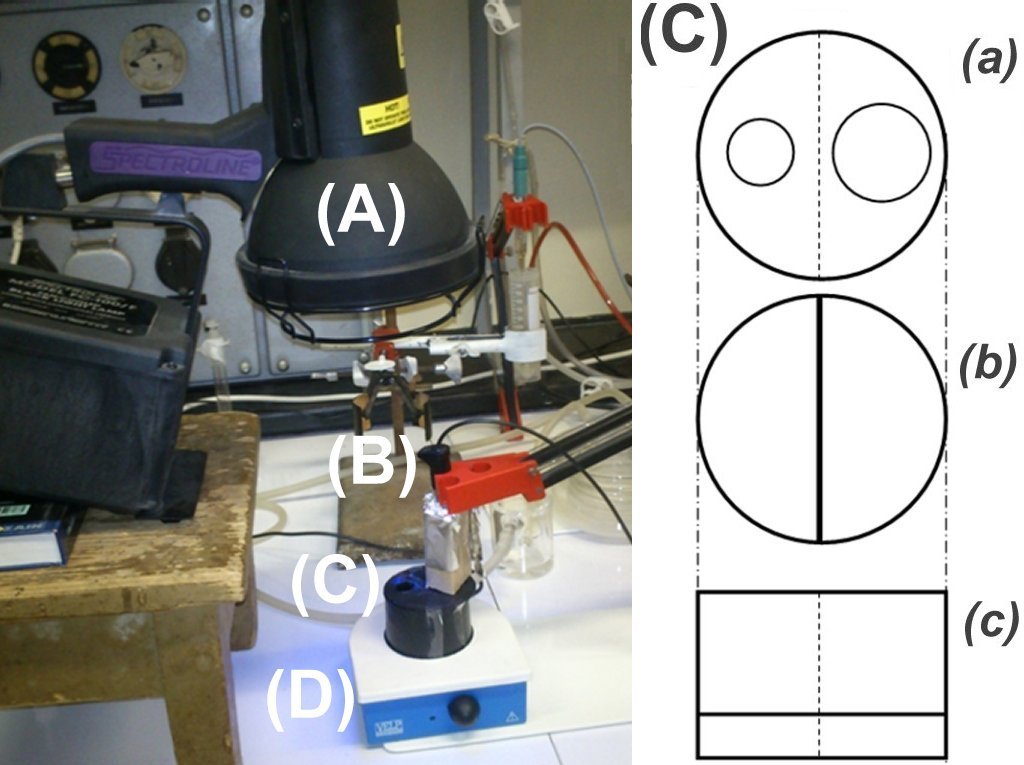
| Chloride ion selective electrodes (B) are light sensitive when irradiated by an intense UV or Vis light (e.g. a Spectroline FC-100/F UV-A lamp (A), or a 500 W NINGBO OALY halogen lamp). This problem can be eliminated by using a specially designed, stirred (D) and calibrated sample holder (C) which spatially separates the illumination and detection sites. The material of the photochemical sample holder (C) is black plastic (poly-propylene). The inside wall has a 10-mm high slit for the stir bar. The cap (a) has a 16-mm diameter whole for the electrode and a larger one for the UV light. The optimal volume of the sample holder is 50 mL. To record the electrode potential, a computer controlled automatic titrator system is used. (a) cap (top view) (b) sample holder (top view) (c) sample holder (side view) |
Top of page